
GORE® TAG® Thoracic Branch Endoprosthesis
First-of-its kind FDA / MDR CE MARK approved device designed for simplified, minimally invasive zone 2 TEVAR procedures. Deliver results without the potential risk and complexity of surgical revascularization.
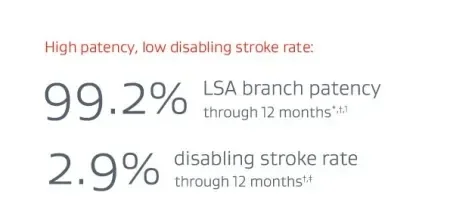
Off-the-shelf endovascular grafts are preferable to traditional surgical options for LSA preservation

Pivotal trial outcome. One year follow-up.
“The GORE® TAG® Thoracic Branch Endoprosthesis has the potential to simplify the treatment of Zone 2 LSA revascularization because it is a single device that can be used in a single procedure.”
Michael Dake, M.D.,
Co-national principal investigator
Senior Vice President, University of Arizona Health Services

From pre-case planning to device procedural consultation, we are by your side to provide support.
- Training needs – Essential technical support with a deep reservoir of product knowledge.
- 8+ years – Average field representatives’ tenure supporting Gore's aortic devices, building on diverse clinical backgrounds.
- Non-commissioned sales force – Our focus is on outcomes.
Contact your Gore Field Sales Associate to learn more.
* 100% freedom from reintervention due to loss of LSA patency.
† Through 12 months.
‡ Data on file 2023; W. L. Gore & Associates, Inc; Flagstaff, AZ.
- Brown J, Gorman J, Sondreaal M, Powis S. Evaluation of the GORE®TAG® Thoracic Branch Endoprosthesis (TBE Device) in the Treatment of Lesions of the Descending Thoracic Aorta (Pivotal): Zone 2. Flagstaff, AZ: W. L. Gore & Associates, Inc; 2021. [Pre-Market Approval Study Report]. MD185099. Rev 1.
![]()
Refer to Instructions for Use at eifu.goremedical.com for a complete description of all applicable indications, warnings, precautions and contraindications for the markets where this product is available. RXOnly
INDICATIONS FOR USE EUROPE: The GORE® TAG® Thoracic Branch Endoprosthesis is intended for endovascular repair of all lesions of the descending thoracic aorta (including isolated lesions, such as aneurysm and traumatic transection, and Type B dissections) while maintaining flow into the left subclavian artery, in patients who have appropriate anatomy.
CONTRAINDICATIONS: The GORE® TAG® Thoracic Branch Endoprosthesis is contraindicated in: Patients with known sensitivities or allergies to the device materials (see Product components), patients who have a condition that threatens to infect the graft and patients with known hypersensitivity to heparin, including those patients who have had a previous incident of Heparin-Induced Thrombocytopenia (HIT) type II.
Product may not be available in all countries.
Please check with your Gore representative for availability.
24AR2142-EN01


